Resources
Sunoptic Surgical brochures and equipment instruction for usage manuals with worldwide support are great benefits to our Physicians, Surgeons, Dentists and other customer communities.
Brochures
Instructions for Use (IFU)
Light Sources
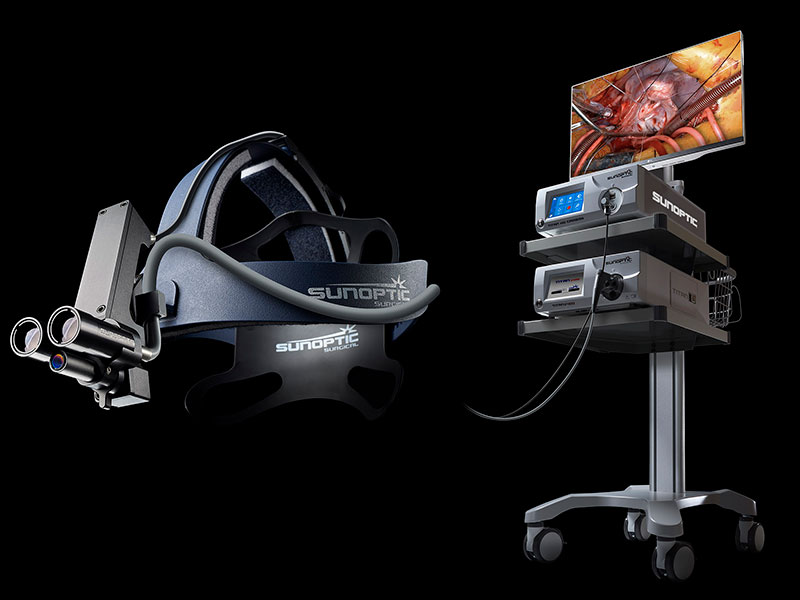
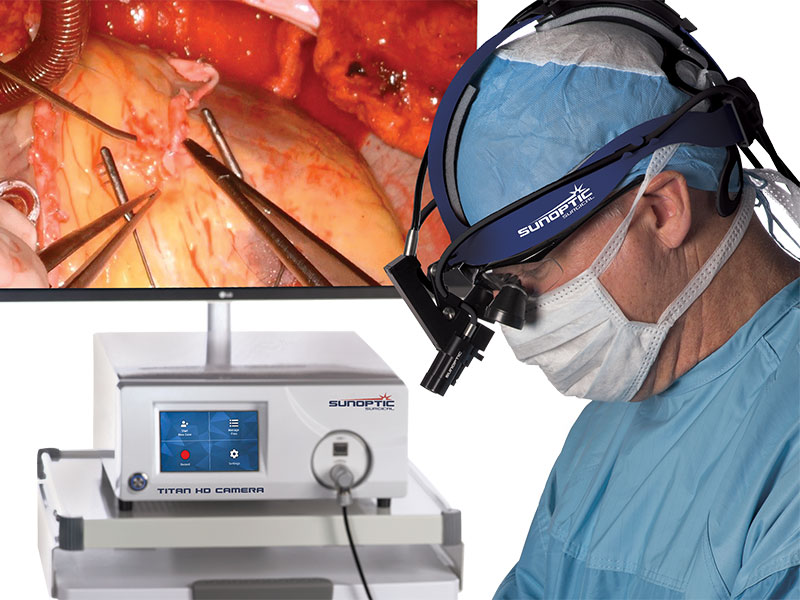
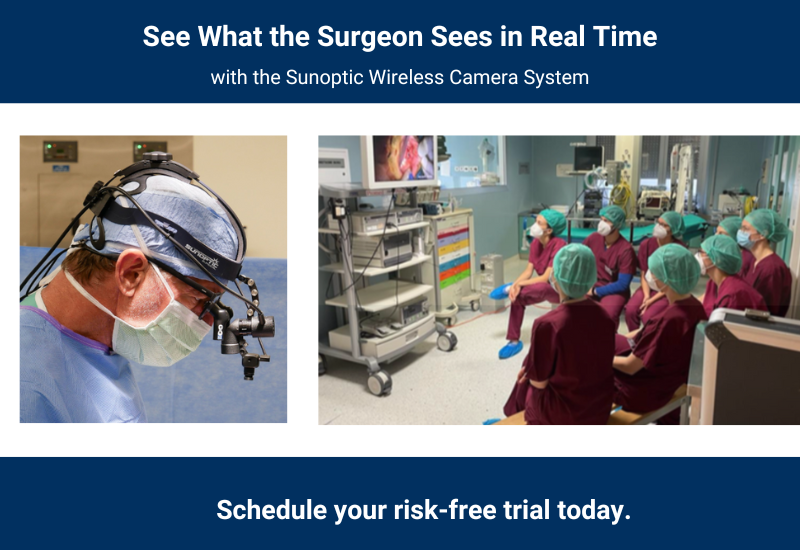
Surgical Headlights
Camera Systems
Cables
Replacement Parts & Accessories
Certifications
Sunoptic Technologies® manufactures Medical Devices in compliance with the current Good Manufacturing Practice (CGMP) requirements set forth in the FDA Quality System Regulation (QSR), Title 21 Part 820 of the Code of Federal Regulations. This QSR helps assure that medical devices are safe and effective for their intended use.
The QSR requires that specifications and controls be established for devices; that devices be designed and manufactured under a quality system to meet these specifications; that finished devices meet these specifications; be correctly installed, checked and serviced; that quality data be analyzed to identify and correct quality problems; and that complaints be processed. Basically, a quality management system must be established to cover design through servicing of a finished device and Sunoptic Technologies® has an effective system in place.
Sunoptic Technologies® is certified to ISO 13485:2016 Medical devices / Quality management systems (requirements for regulatory purposes), MDSAP certified- also requirements for regulatory purposes, as well as CE Certified (for EU Requirements). Our focus is on the customer and our continual improvement of the quality management system will enhance customer satisfaction.